AMI Cardiogenic Shock
Clinical Evidence

Best Practice Protocols Include 1-4
- Identify CS early and Impella pre-PCI < 90 minutes
- Aggressive down-titration of inotropes
- Identify RV dysfunction early and support
- Identify inadequate LV support and escalate
- Systematic use of RHC to guide therapy

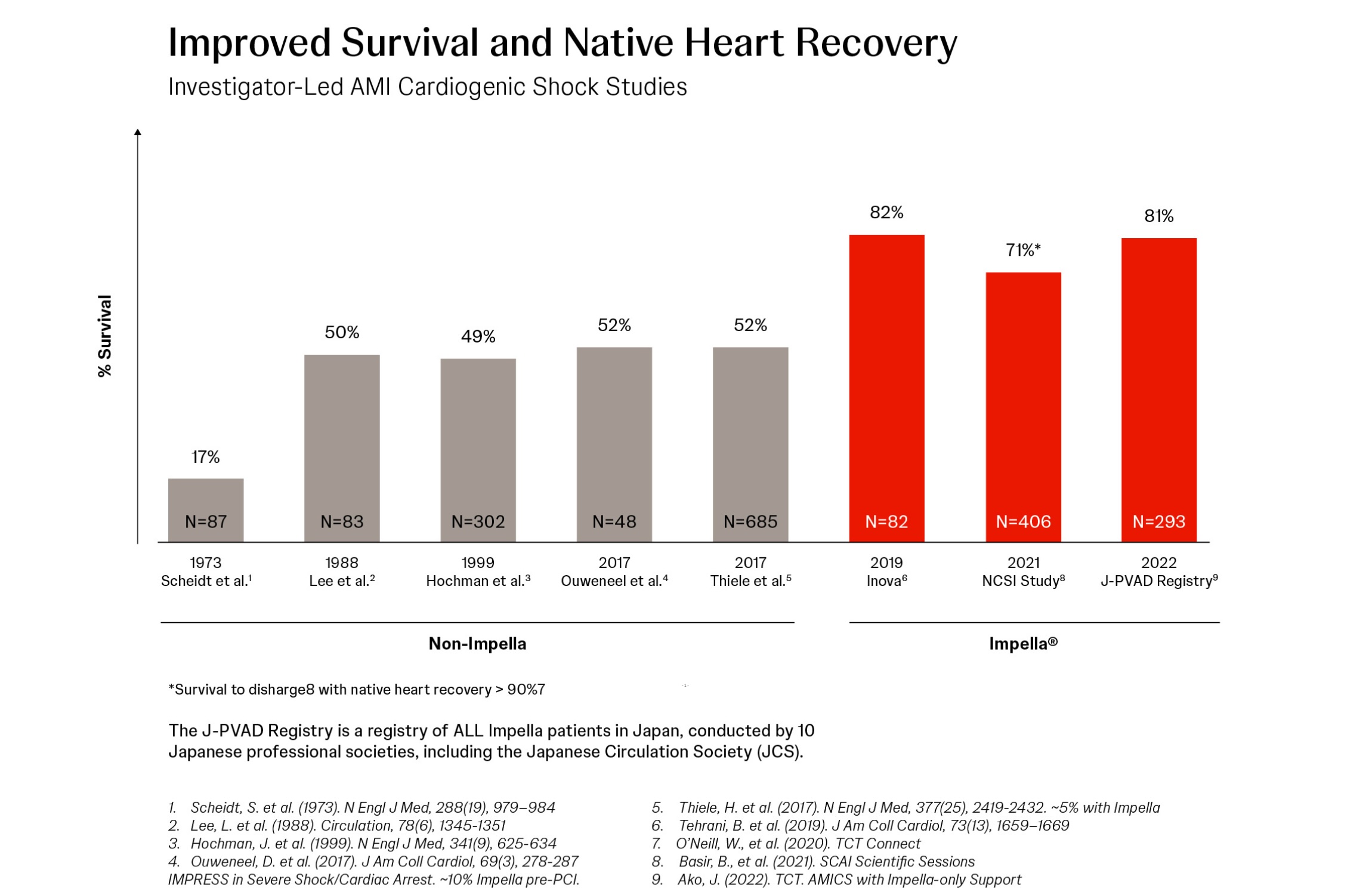
81% Survival at 30 Days in a Multi-Center, Multi-Society Study of Impella®-Supported AMI Cardiogenic Shock Patients
Junya Ako, MD, presents data from 593 consecutive patients with AMI cardiogenic shock (AMICS) supported with Impella heart pumps at 109 centers in Japan between October 2017 (when Impella heart pump was introduced in Japan) through January 2020.
Impella Clinical Evidence has Established Safety and Effectiveness and Validated Best Practices


RECOVER IV Randomized Controlled Trial
Upcoming trial to assess whether Impella pre-PCI is superior to PCI without Impella in patients with AMI cardiogenic shock.
References
- Tehrani, B. et al. (2019). J Am Coll Cardiol, 73(13), 1659–1669.
- O’Neill, W., et al. (2020). TCT Connect.
- Basir, B., et al. (2021). SCAI Scientific Sessions.
- Ako, J. (2022). TCT. AMICS with Impella-only Support.
NPS-1187
