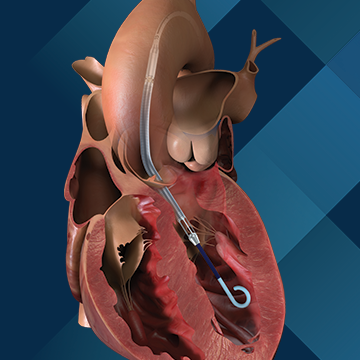
First Completed RCT of Impella® in AMICS
The FDA has granted Impella a Premarket Approval (PMA) for high-risk PCI, cardiogenic shock and right heart failure.
- 1,200+
Peer-reviewed clinical studies have published on Impella, including the PROTECT II randomized controlled trial
- 13
Clinical society guidelines include Impella heart pumps
- 300,000+
Patients have been treated with Impella heart pumps
Early Utilization of Mechanical Circulatory Support in Acute Myocardial Infarction Complicated by Cardiogenic Shock
Mir B. Basir, DO, discusses best practices from the National Cardiogenic Shock Initiative (NCSI) Study.

For Interventional Cardiologists
Treat high-risk PCI, cardiogenic shock and right heart failure patients
For Cardiac and Vascular Surgeons
Escalate, stabilize and recover native organs with minimally-invasive surgical techniques

For Nurses
Use calculators, watch quick skills videos and learn patient management best practices

For Fellows
Advance your knowledge and develop skills to treat complex PCI patients

For Referring Providers
Are you caring for patients with heart disease? Learn more about how Impella heart pumps work, the benefits of Protected PCI with Impella and explore patient identification resources.
References
- Møller, J., et al. (2024). Microaxial Flow Pump or Standard Care in Infarct-Related Cardiogenic Shock. N Engl J Med.
NPS-1309


